
R&D at 20/30 Labs is made up of a dynamic team with specialties in Microbiology (PhD, Mphil), Decontamination, Molecular Biology, Parasitology, Virology, Clinical, Biochemistry, Botany, and Life Sciences. Our BSL II facility is fully equipped with state-of-the-art equipment including a Class 5 clean room, ICP-MS, 3D printing, PCR, liquid handling automation, medical packing systems, air sampling and cascade impaction devices, and microorganism preservation technology.
We offer a wide range of microbiological validation services for medical devices including decontamination assurance, testing the design of decontamination processes, diagnosis of contamination events, performance qualification, shelf-life testing, and proof of concept.

Validation of cleaning, disinfection efficacy and sterility assurance levels [HTM 01-01, ISO 15883].

Early warning detection systems of the presence of infection

How 20/30 Labs classify medical face masks (Type I, Type II or Type IIR) as per BS EN 14683:2019.
How 20/30 Labs undertake ISO classification and microbiological sampling of cleanrooms.

A medical equipment manufacturing company had been supplied with a filter product, with the filter material sealed in plastic housing with connectors on either end.
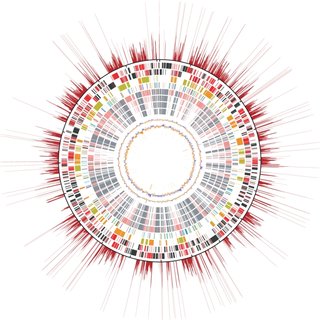
Whole genome sequencing of two bacterial isolates to determine a source outbreak.